The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → c...

Chemistry, 20.01.2020 03:31 tatianaflores9040
The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → cacl_2 (aq) + h_2 (g)
if 500.0 g of calcium are added to an excess of hci, what volume of h_2 is produced?
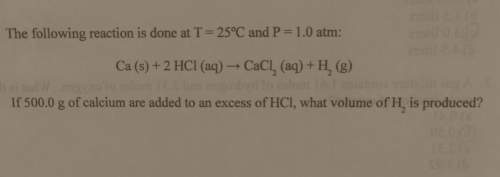

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

You know the right answer?
Questions

Chemistry, 29.04.2021 08:40







History, 29.04.2021 08:40

Mathematics, 29.04.2021 08:40

Mathematics, 29.04.2021 08:40

Mathematics, 29.04.2021 08:40



Mathematics, 29.04.2021 08:40




Mathematics, 29.04.2021 08:40


English, 29.04.2021 08:40




