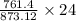Chemistry, 19.01.2020 09:31 haileestulley
(40 points) complete, balance, compute the amounts of the products assuming 100% yield. 12g chlorine + 24g ferric iodide.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
(40 points) complete, balance, compute the amounts of the products assuming 100% yield. 12g chlorine...
Questions



English, 05.11.2020 06:50




Biology, 05.11.2020 06:50

Computers and Technology, 05.11.2020 06:50



Geography, 05.11.2020 06:50

Biology, 05.11.2020 06:50


English, 05.11.2020 06:50






Chemistry, 05.11.2020 06:50

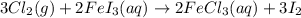
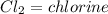
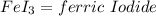
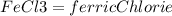
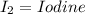
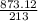
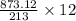
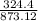
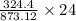 of FeCl3
of FeCl3