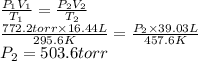Chemistry, 18.01.2020 06:31 joshlynn52
Acylinder fitted with a movable piston and filled with a gas has a volume of 16.44 liters at 22.4°c when the applied pressure is 772.2 torr.
the temperature of the oil bath surrounding the piston was increased to 184.4°c, and the pressure on the piston was changed. careful measurement now gave a value of 39.03 liters for the new volume.
what is the final pressure in the cylinder, expressed in torr?
a) 494.0 torr
b) 503.6 torr
c) 1184 torr
d) 1207 torr
e) 6295 torr
with explanation .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Acylinder fitted with a movable piston and filled with a gas has a volume of 16.44 liters at 22.4°c...
Questions

Mathematics, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30




Mathematics, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30



Business, 15.10.2019 20:30

Mathematics, 15.10.2019 20:30

History, 15.10.2019 20:30