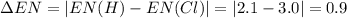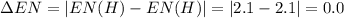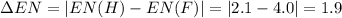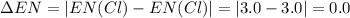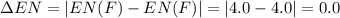Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

You know the right answer?
Consider the following electronegativity values:
h = 2.1, cl = 3.0, f = 4.0
which mole...
h = 2.1, cl = 3.0, f = 4.0
which mole...
Questions


Mathematics, 21.04.2021 01:00




Chemistry, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00



Mathematics, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00

History, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00

Advanced Placement (AP), 21.04.2021 01:00



Mathematics, 21.04.2021 01:00

Chemistry, 21.04.2021 01:00

English, 21.04.2021 01:00