
Chemistry, 18.01.2020 03:31 kymberlyasher
Which of the following gases would you expect to deviate significantly from ideal behavior: he, o2, h2, h2o, n2, hcl, or nh3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Which of the following gases would you expect to deviate significantly from ideal behavior: he, o2,...
Questions


Spanish, 25.09.2019 14:10

Chemistry, 25.09.2019 14:10

Mathematics, 25.09.2019 14:10

English, 25.09.2019 14:10


History, 25.09.2019 14:10


Mathematics, 25.09.2019 14:10



Social Studies, 25.09.2019 14:10


History, 25.09.2019 14:10

Biology, 25.09.2019 14:10

History, 25.09.2019 14:10

History, 25.09.2019 14:10


Mathematics, 25.09.2019 14:10

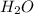 , HCl, and
, HCl, and 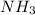 are all polar molecules therefore, these tend to deviate from ideal behavior.
are all polar molecules therefore, these tend to deviate from ideal behavior.

