

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Avoltaic cell consists of a hg/hg22+ electrode (e°= 0.85 v) and a sn/sn2+ electrode (e°= –0.14 v). c...
Questions

Arts, 22.06.2021 21:00

History, 22.06.2021 21:00



Mathematics, 22.06.2021 21:00


English, 22.06.2021 21:00

Spanish, 22.06.2021 21:00


Chemistry, 22.06.2021 21:10


English, 22.06.2021 21:10

History, 22.06.2021 21:10

Mathematics, 22.06.2021 21:10



Biology, 22.06.2021 21:10

Mathematics, 22.06.2021 21:10

Mathematics, 22.06.2021 21:10

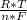 *ln(Q)
*ln(Q)![\frac{[C]^{c}*[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0460/2641/2f4bf.png) In this case [C] and [D], they refer to the partial pressures, also known as molar concentrations in the case of gases or ions in solution, for the reaction products. [A] and [B] are also partial pressures but in the case of reagents. The exponents refer to the amount of moles that make up each substance that is participating in the reaction. Substances that are in a solid state have a unit concentration (that is, their value is 1), so they do not appear in Q.
In this case [C] and [D], they refer to the partial pressures, also known as molar concentrations in the case of gases or ions in solution, for the reaction products. [A] and [B] are also partial pressures but in the case of reagents. The exponents refer to the amount of moles that make up each substance that is participating in the reaction. Substances that are in a solid state have a unit concentration (that is, their value is 1), so they do not appear in Q.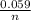 *ln(Q) Equation (A)
*ln(Q) Equation (A)![\frac{[Sn^{2+}] }{[Hg_{2} ^{+2} ]}](/tpl/images/0460/2641/af92b.png) where [Hg₂²⁺] is 0.24 M
where [Hg₂²⁺] is 0.24 M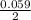 *ln
*ln![\frac{[Sn]^{2+} }{0.24}](/tpl/images/0460/2641/8f497.png)



