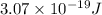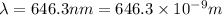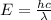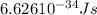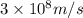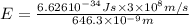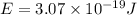When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon of approximate wavelength 646.3 nm is emitted. the energy difference between these 2p and 2s orbitals is: . 3.07 ã 10^â28 jb. 3.07 ã 10^â19 jc. 3.07 ã 10^â17 jd. 1.28 ã 10^â31 je. none of these

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

You know the right answer?
When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon...
Questions

Computers and Technology, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50


Geography, 13.01.2021 17:50

Social Studies, 13.01.2021 17:50



English, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50

Mathematics, 13.01.2021 17:50

Computers and Technology, 13.01.2021 17:50



Social Studies, 13.01.2021 17:50



Chemistry, 13.01.2021 17:50

Physics, 13.01.2021 17:50