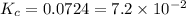Chemistry, 18.01.2020 00:31 honeylebling
Mercury(ii) oxide decomposes to form mercury and oxygen, like this: (s)(l)(g) at a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(ii) oxide, mercury, and oxygen at equilibrium has the following composition:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
Mercury(ii) oxide decomposes to form mercury and oxygen, like this: (s)(l)(g) at a certain temperat...
Questions


Mathematics, 14.04.2021 05:00

Mathematics, 14.04.2021 05:00


Social Studies, 14.04.2021 05:00

Mathematics, 14.04.2021 05:00

Mathematics, 14.04.2021 05:00



Mathematics, 14.04.2021 05:00

Arts, 14.04.2021 05:00

Chemistry, 14.04.2021 05:00

Social Studies, 14.04.2021 05:00

History, 14.04.2021 05:00

Mathematics, 14.04.2021 05:00



Mathematics, 14.04.2021 05:00

Social Studies, 14.04.2021 05:00

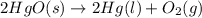
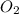 22.7 g
22.7 g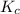 for this reaction. Round your answer to 2 significant digits.
for this reaction. Round your answer to 2 significant digits.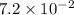
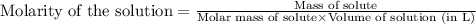
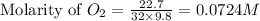
![K_c=[O_2]](/tpl/images/0460/0182/99fa8.png)