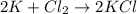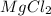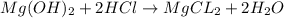Chemistry, 07.10.2019 17:50 aidanfbussiness
Check my semester exam ! i have a flight to must submit !
question 1 (multiple choice worth 2 points)
[07.06]what is the major difference between nuclear and chemical reactions?
only chemical reactions release energy.
only chemical reactions release gamma rays.
only nuclear reactions involve the exchange of electrons.
i picked: only nuclear reactions change the identity of an element.
question 2 (multiple choice worth 2 points)
[07.03]when water is added to an erlenmeyer flask and then sealed, phase equilibrium occurs. which answer explains the resulting dynamic equilibrium?
rate of evaporation = rate of precipitation
i picked: rate of condensation = rate of evaporation
moles of vapor = moles of liquid
moles of vapor = moles of solid
question 3 (multiple choice worth 2 points)
[05.01]which phase of matter is made up of particles that are packed closely together, with both a definite shape and a definite volume?
gas
liquid
plasma
i picked: solid
question 4 (multiple choice worth 2 points)
[06.02]when sulfuric acid is poured on sugar the volume of sugar expands and the sugar turns black and becomes very hot. which of the following best describes this reaction?
endothermic
i picked: exothermic
decomposition
synthesis
question 5 (multiple choice worth 2 points)
[07.01]which of the following is not true of acids?
acids are corrosive.
i picked: acids feel slippery.
acids have a sour taste.
acids have more hydronium ions than hydroxide ions.
question 6 (multiple choice worth 2 points)
[06.04]according to the collision theory, what is the best explanation for why a higher temperature makes a reaction go faster?
there are fewer collisions per minute.
it increases the distance between the particles.
it decreases the average kinetic energy of the particles.
i picked: it increases the average kinetic energy and number of collisions per minute.
question 7 (multiple choice worth 2 points)
[07.02]which of the following represents pure water?
[h3o+] > [oh-]
[h3o+] < [oh-]
i picked: [h3o+] = [oh-]
[h3o+] [oh-] = 14
question 8 (multiple choice worth 2 points)
[07.06]which of the following types of radiation can penetrate the most deeply into your body?
alpha rays
beta rays
i picked: gamma rays
proton rays
question 9 (multiple choice worth 2 points)
[07.05]in the chemical reaction shown below, which element was oxidized?
2k + cl2 yields 2kcl
i picked: k, because it lost one electron
k, because it gained one electron
cl, because it lost one electron
cl, because it gained one electron
question 10 (multiple choice worth 2 points)
[06.02]on an exothermic potential energy diagram, what is true for the products?
the products are at the same energy level as the reactants.
i picked: the products are at a lower energy level than the reactants.
the products are at a higher energy level than the reactants.
the products do not have energy.
question 11 (multiple choice worth 2 points)
[07.02]what is the salt formed in the following neutralization reaction?
mg(oh)2 + hcl yields h2o + mgcl2
mg(oh)2
hcl
h2o
i picked: mgcl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Check my semester exam ! i have a flight to must submit !
question 1 (multiple choice...
question 1 (multiple choice...
Questions

Arts, 30.03.2021 05:50

Mathematics, 30.03.2021 05:50

Physics, 30.03.2021 05:50

Mathematics, 30.03.2021 05:50

History, 30.03.2021 05:50


Mathematics, 30.03.2021 05:50

Mathematics, 30.03.2021 05:50

Social Studies, 30.03.2021 05:50

Mathematics, 30.03.2021 05:50

History, 30.03.2021 05:50

Mathematics, 30.03.2021 05:50

Mathematics, 30.03.2021 06:00

Chemistry, 30.03.2021 06:00

Health, 30.03.2021 06:00



English, 30.03.2021 06:00


Business, 30.03.2021 06:00

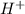 ion is their aqueous solution.They taste sour and corrosive in nature.
ion is their aqueous solution.They taste sour and corrosive in nature.![[H_3O^+]=[OH^-]](/tpl/images/0297/5506/f28a0.png)
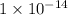
![pH=-\log[H_3O^+]=7](/tpl/images/0297/5506/4c7e1.png)
![[H_3O^+]=1\times 10^{-7}](/tpl/images/0297/5506/e8ae8.png)
![K_w=1\times 10^{-14}=[H_3O^+][OH^-]](/tpl/images/0297/5506/3cca3.png)