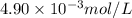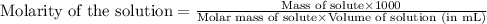Achemist prepares a solution of copper(ii) fluoride by measuring out of copper(ii) fluoride into a volumetric flask and filling the flask to the mark with water. calculate the concentration in of the chemist's copper(ii) fluoride solution. round your answer to significant digits. initial knowledge check answers

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Achemist prepares a solution of copper(ii) fluoride by measuring out of copper(ii) fluoride into a v...
Questions


Mathematics, 09.11.2020 19:40







English, 09.11.2020 19:40

Social Studies, 09.11.2020 19:40

Mathematics, 09.11.2020 19:40

Mathematics, 09.11.2020 19:40

History, 09.11.2020 19:40




Chemistry, 09.11.2020 19:40

Social Studies, 09.11.2020 19:40

Mathematics, 09.11.2020 19:40

Chemistry, 09.11.2020 19:40