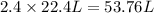Chemistry, 16.01.2020 22:31 BreBreDoeCCx
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced according to the balanced chemical equation below:
caco3(s) + 2hc1(aq) 4 cac12(aq) +h20(1) + co2(g)
imagine mixing 2.5 moles of calcium carbonate with 4.8 moles of hydrochloric acid. calculate the number of moles of caco3 and hcl used in the intro activity. determine the limiting reactant and use the ideal gas law to estimate the max volume of co2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions

Mathematics, 20.04.2021 04:10

History, 20.04.2021 04:10


Mathematics, 20.04.2021 04:10




Mathematics, 20.04.2021 04:10

History, 20.04.2021 04:10

Mathematics, 20.04.2021 04:10


History, 20.04.2021 04:10



English, 20.04.2021 04:10

English, 20.04.2021 04:10

Mathematics, 20.04.2021 04:10



Mathematics, 20.04.2021 04:10

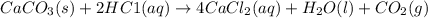
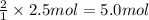 of HCl
of HCl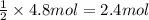 of calcium carbonate
of calcium carbonate