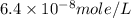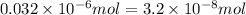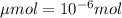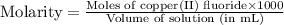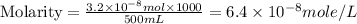Chemistry, 16.01.2020 20:31 starfox5454
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii) fluoride into a 500 ml volumetric flask and filling the flask to the mark with water. calculate the concentration in mol/l of the chemist's copper(ii) fluoride solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Achemist prepares a solution of copper(ii) fluoride (cuf2) by measuring out 0.032 µmol of copper(ii)...
Questions

Social Studies, 22.03.2021 22:00





Mathematics, 22.03.2021 22:00

Mathematics, 22.03.2021 22:00


Mathematics, 22.03.2021 22:00



Mathematics, 22.03.2021 22:00

World Languages, 22.03.2021 22:00

Mathematics, 22.03.2021 22:00