
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperature of 25 degrees celcius. if all this oxygen were converted to ozone at the same temperature and pressure, what would be the volume of the ozone? balanced equation: 3o2 → 2o3a. 6.5lb. 2.8lc. 8.1ld. 7.4l

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperat...
Questions



Health, 03.05.2021 18:20

World Languages, 03.05.2021 18:20

Mathematics, 03.05.2021 18:20

Mathematics, 03.05.2021 18:20

Mathematics, 03.05.2021 18:20

Biology, 03.05.2021 18:20

Mathematics, 03.05.2021 18:20


English, 03.05.2021 18:20









Mathematics, 03.05.2021 18:20

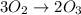
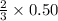 moles of ozone
moles of ozone

