Abeaker has 0.2 m of na2so4. what will be the concentration of sodium and sulfate ions?
[na+]...

Chemistry, 16.01.2020 17:31 brummy309506
Abeaker has 0.2 m of na2so4. what will be the concentration of sodium and sulfate ions?
[na+] = 0.4 m, [so42-] = 0.2 m
[na+] = 0.2 m, [so42-] = 0.2 m
[na+] = 0.4 m, [so42-] = 0.4 m
[na+] = 0.2 m, [so42-] = 0.4 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Questions

Mathematics, 30.06.2019 14:30


History, 30.06.2019 14:30


History, 30.06.2019 14:30




English, 30.06.2019 14:30



English, 30.06.2019 14:30


Social Studies, 30.06.2019 14:30


History, 30.06.2019 14:30




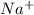 ] = 0.4 M, [
] = 0.4 M, [ ] = 0.2 M
] = 0.2 M


