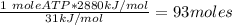Chemistry, 16.01.2020 05:31 JayLiz1737
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of atp that can be synthesized from adp from the breakdown of one mole of glucose. c6h12o6(s) + 6 o2(g) → 6 co2(g) + 6 h2o(l) δg° = −2880 kj/mol adp + h3po4 → atp + h2o δg° = 31 kj/mol webassign will check your answer for the correct number of significant figures. moles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of...
Questions

Spanish, 06.10.2020 14:01

History, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01


Geography, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Chemistry, 06.10.2020 14:01





Computers and Technology, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01

Biology, 06.10.2020 14:01


Law, 06.10.2020 14:01


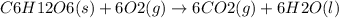 ΔG°=-2880 KJ/mol
ΔG°=-2880 KJ/mol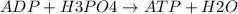 ΔG°=31 KJ/mol
ΔG°=31 KJ/mol