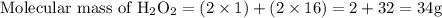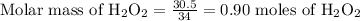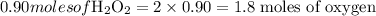Chemistry, 16.01.2020 05:31 unknown9263
How many moles of oxygen atoms are present in 30.5 grams of hydrogen peroxide (h2o2)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3


Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1

You know the right answer?
How many moles of oxygen atoms are present in 30.5 grams of hydrogen peroxide (h2o2)?...
Questions




Computers and Technology, 30.03.2020 21:02


Mathematics, 30.03.2020 21:02

English, 30.03.2020 21:02





Chemistry, 30.03.2020 21:02

English, 30.03.2020 21:02

History, 30.03.2020 21:02

Mathematics, 30.03.2020 21:02

English, 30.03.2020 21:02