
Chemistry, 15.01.2020 22:31 Mariaisagon8446
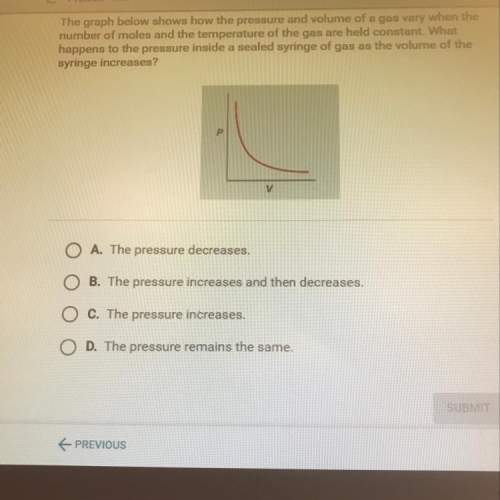
The graph below shows how the pressure and volume of a gas vary when the
number of moles and the temperature of the gas are held constant. what
happens to the pressure inside a sealed syringe of gas as the volume of the
syringe increases?
(sorry the picture sucks)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
The graph below shows how the pressure and volume of a gas vary when the
number of moles and t...
number of moles and t...
Questions

English, 29.06.2021 05:40



Mathematics, 29.06.2021 05:40

English, 29.06.2021 05:40



Mathematics, 29.06.2021 05:40



Mathematics, 29.06.2021 05:40


Mathematics, 29.06.2021 05:40

Arts, 29.06.2021 05:40

Chemistry, 29.06.2021 05:40



History, 29.06.2021 05:40


Mathematics, 29.06.2021 05:40



