
An unknown oxide of mercury decomposes when heated to form mercury metal and oxygen gas. when a 1.048 g sample of this unknown is heated, 0.971 g of mercury remains.
a. calculate the moles of mercury and moles of oxygen in the compound.
b. what is the empirical formula of this oxide?
2. a side reaction in today

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
You know the right answer?
An unknown oxide of mercury decomposes when heated to form mercury metal and oxygen gas. when a 1.04...
Questions



Biology, 26.08.2019 04:10

Mathematics, 26.08.2019 04:10


Mathematics, 26.08.2019 04:10

History, 26.08.2019 04:10



Mathematics, 26.08.2019 04:10

English, 26.08.2019 04:10

English, 26.08.2019 04:10

Chemistry, 26.08.2019 04:10




Mathematics, 26.08.2019 04:10


Mathematics, 26.08.2019 04:10

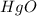
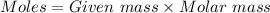
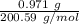 = 0.00484 mol.
= 0.00484 mol.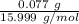 = 0.00481 mol.
= 0.00481 mol.

