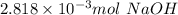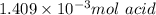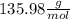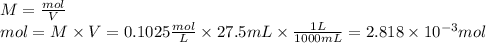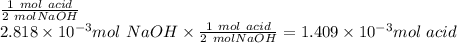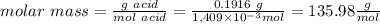Chemistry, 15.01.2020 19:31 eyeneedalife
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid was then titrated with 0.1025m naoh solution. the second equivalence point showed the sharpest change in ph, and so it was used to determine the molar mass of the unknown acid. the volume of naoh needed to reach the equivalence point was 27.5 ml.
a. calculate the number of moles of naoh used in the titration to reach the second equivalence point.
b. calculate the number of moles of diprotic acid, based on the fact that we are examining the second equivalence point.
c. calculate the molar mass of the diprotic acid.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid w...
Questions


Biology, 23.07.2019 08:00


History, 23.07.2019 08:00

History, 23.07.2019 08:00

History, 23.07.2019 08:00

Mathematics, 23.07.2019 08:00

Geography, 23.07.2019 08:00

English, 23.07.2019 08:00



History, 23.07.2019 08:00

History, 23.07.2019 08:00

Mathematics, 23.07.2019 08:00


History, 23.07.2019 08:00


Arts, 23.07.2019 08:00


Social Studies, 23.07.2019 08:00