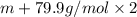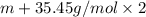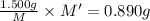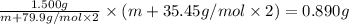Chemistry, 15.01.2020 19:31 whitakers87
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical formula xci2. the dibromide is completely converted to the dichloride when it is heated in a stream of chlorine according to the reaction $$xbr2 + cl2 → xci2 + br2$$ when 1.500 g xbr2 is treated, 0.890 g xci2 results. (a) calculate the atomic mass of the element x. (b) by reference to a list of the atomic masses of the elements, identify the element x.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical fo...
Questions


Chemistry, 23.05.2020 19:03

Mathematics, 23.05.2020 19:03

Mathematics, 23.05.2020 19:03

Social Studies, 23.05.2020 19:03


Chemistry, 23.05.2020 19:04

Mathematics, 23.05.2020 19:04


Mathematics, 23.05.2020 19:04

English, 23.05.2020 19:04

Mathematics, 23.05.2020 19:04









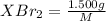 mol
mol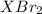 gives 1 mole of
gives 1 mole of 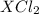 . then
. then 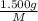 mol of [tex[CBr_2[/tex] will give:
mol of [tex[CBr_2[/tex] will give: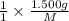 mol=\frac{1.500 g}{M}[/tex] mol[/tex] of
mol=\frac{1.500 g}{M}[/tex] mol[/tex] of