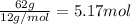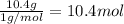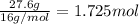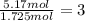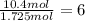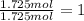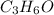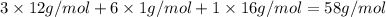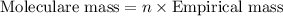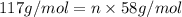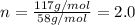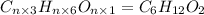Chemistry, 15.01.2020 04:31 alondrasanchezvillan
Compound x, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. a careful analysis showed that compound x contains 62% carbon and 10.4% hydrogen. no nitrogen or halogen was found.
a. compute an empirical formula for compound x.
express your answer as a condensed structural formula.
b. a molecular weight determination showed that compound x has a molecular weight of approximately 117. find the molecular formula of compound x.
express your answer as a condensed structural formula.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
Compound x, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. a c...
Questions


Mathematics, 21.12.2020 23:00






Mathematics, 21.12.2020 23:00

History, 21.12.2020 23:00


Biology, 21.12.2020 23:00



Mathematics, 21.12.2020 23:00

Physics, 21.12.2020 23:00

Biology, 21.12.2020 23:00

History, 21.12.2020 23:00


Mathematics, 21.12.2020 23:00

Mathematics, 21.12.2020 23:00

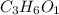 .
.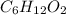 .
.