
Chemistry, 15.01.2020 03:31 angeljohnson2081
Sodium hydrogen carbonate nahco3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid hcl, which the stomach secretes to digest food. drinking a glass of water containing dissolved nahco3 neutralizes excess hcl through this reaction: hcl(aq)+nahco3(aq)→nacl(aq)+h2o(l)+ co2(g)
the co2gas produced is what makes you burp after drinking the solution. suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.ml of a 0.089m hcl solution. what mass of nahco3 would she need to ingest to neutralize this much hcl? be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
Sodium hydrogen carbonate nahco3, also known as sodium bicarbonate or "baking soda", can be used to...
Questions





Mathematics, 23.07.2019 00:00



Chemistry, 23.07.2019 00:00

History, 23.07.2019 00:00











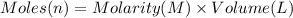
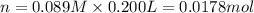 of HCL
of HCL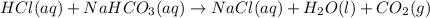
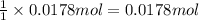 of sodium bicarbonate
of sodium bicarbonate

