Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
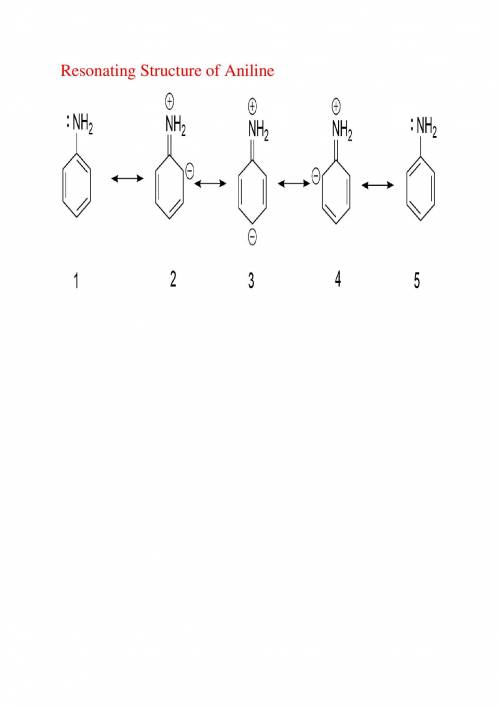
As the extent of electron delocalization into the ring increases, the geometry at nitrogen flattens....
Questions

Mathematics, 05.03.2021 02:10

Chemistry, 05.03.2021 02:10


Mathematics, 05.03.2021 02:10

Mathematics, 05.03.2021 02:10




Mathematics, 05.03.2021 02:10


English, 05.03.2021 02:10


Mathematics, 05.03.2021 02:10

Mathematics, 05.03.2021 02:10

Chemistry, 05.03.2021 02:10





Biology, 05.03.2021 02:10