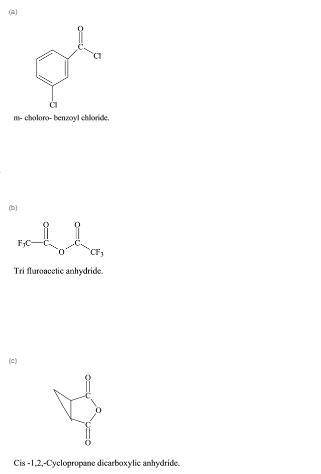
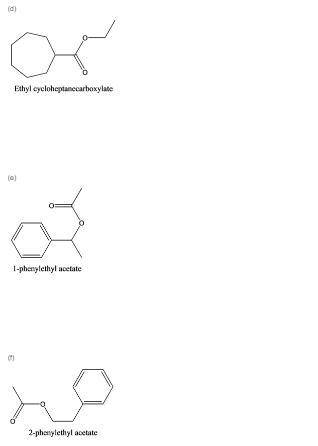
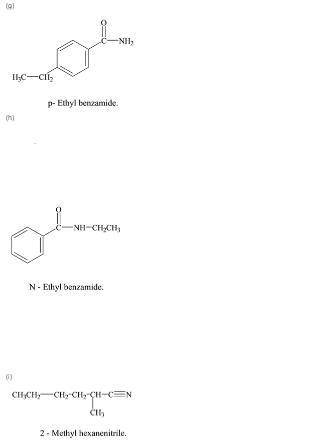
Write a structural formula for each of the following compounds:
a. m-chlorobenzoyl chl...

Chemistry, 14.01.2020 21:31 livingfamyboys35
Write a structural formula for each of the following compounds:
a. m-chlorobenzoyl chloride
b. trifluoroacetic anhydride
c. cis-1,2-cyclopropanedicarboxylic anhydride
d. ethyl cycloheptanecarboxylate
e. 1-phenylethyl acetate
f. 2-phenylethyl acetate
g. p-ethylbenzamide
h. n-ethylbenzamide
i. 2-methylhexanenitrile

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Questions

World Languages, 24.11.2019 05:31

Spanish, 24.11.2019 05:31

Geography, 24.11.2019 05:31



Chemistry, 24.11.2019 05:31



Mathematics, 24.11.2019 05:31


Spanish, 24.11.2019 05:31



History, 24.11.2019 05:31

Geography, 24.11.2019 05:31

English, 24.11.2019 05:31


Mathematics, 24.11.2019 05:31