
Chemistry, 14.01.2020 05:31 JAYDENJONES0111
A2.241-g sample of nickel reacts with oxygen to form 2.852 g of the metal oxide.
calculate the empirical formula for oxide.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
A2.241-g sample of nickel reacts with oxygen to form 2.852 g of the metal oxide.
calculate the...
calculate the...
Questions

Physics, 21.02.2020 23:31





Mathematics, 21.02.2020 23:31

Biology, 21.02.2020 23:31









Mathematics, 21.02.2020 23:31



Mathematics, 21.02.2020 23:32

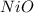
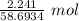 = 0.03818 mol
= 0.03818 mol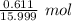 = 0.03818 mol
= 0.03818 mol

