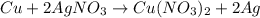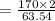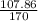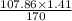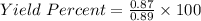Chemistry, 13.01.2020 05:31 davelopez979
In the copper – silver nitrate lab copper medals and silver nitrate solution reacted to produce silver metal and copper (ii) nitrate in solution. a student placed a copper wire with a mass of 2.93 g in the reaction test tube. the silver nitrate solution contained 1.41 g of silver nitrate. he obtained .87 g of silver metal. calculate the percent yield of silver.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
In the copper – silver nitrate lab copper medals and silver nitrate solution reacted to produce silv...
Questions

Mathematics, 26.01.2021 07:20

History, 26.01.2021 07:20

Mathematics, 26.01.2021 07:20

Social Studies, 26.01.2021 07:20

Mathematics, 26.01.2021 07:20

English, 26.01.2021 07:20


Mathematics, 26.01.2021 07:20

English, 26.01.2021 07:20


Chemistry, 26.01.2021 07:20

Mathematics, 26.01.2021 07:20

English, 26.01.2021 07:20

English, 26.01.2021 07:20

Chemistry, 26.01.2021 07:20


Biology, 26.01.2021 07:20



Mathematics, 26.01.2021 07:20