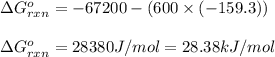Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine.
the data refer t...

Chemistry, 11.01.2020 04:31 smariedegray
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine.
the data refer to 298 k.
so2(g) + cl2(g) --> so2cl2(g)
substance: so2(g) + cl2(g) -> so2cl2(g)
delta h� f (kj/mol): �296.8 0�364.0
delta g� f (kj/mol): �300.1 0 �320.0
delta s�(j/kmol): 248.2 223.0 311.9
what is the value of g� for this reaction at 600 k?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Questions

History, 16.01.2020 00:31

English, 16.01.2020 00:31

Chemistry, 16.01.2020 00:31

Biology, 16.01.2020 00:31

History, 16.01.2020 00:31




Mathematics, 16.01.2020 00:31

History, 16.01.2020 00:31

Mathematics, 16.01.2020 00:31




Computers and Technology, 16.01.2020 00:31





Social Studies, 16.01.2020 00:31

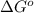 of the reaction is 28.38 kJ/mol
of the reaction is 28.38 kJ/mol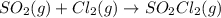
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f_{(product)}]-\sum [n\times \Delta H^o_f_{(reactant)}]](/tpl/images/0450/5212/72c39.png)
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(SO_2Cl_2(g))})]-[(1\times \Delta H^o_f_{(SO_2(g))})+(1\times \Delta H^o_f_{(Cl_2(g))})]](/tpl/images/0450/5212/6dcd3.png)
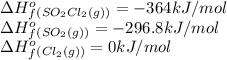
![\Delta H^o_{rxn}=[(1\times (-364))]-[(1\times (-296.8))+(1\times 0)]=-67.2kJ/mol=-67200J/mol](/tpl/images/0450/5212/d9b21.png)
![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_f_{(product)}]-\sum [n\times \Delta S^o_f_{(reactant)}]](/tpl/images/0450/5212/3b842.png)
![\Delta S^o_{rxn}=[(1\times \Delta S^o_{(SO_2Cl_2(g))})]-[(1\times \Delta S^o_{(SO_2(g))})+(1\times \Delta S^o_{(Cl_2(g))})]](/tpl/images/0450/5212/26209.png)

![\Delta S^o_{rxn}=[(1\times 311.9)]-[(1\times 248.2)+(1\times 223.0)]=-159.3J/Kmol](/tpl/images/0450/5212/b641b.png)
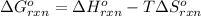
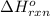 = standard enthalpy change of the reaction =-67200 J/mol
= standard enthalpy change of the reaction =-67200 J/mol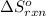 = standard entropy change of the reaction =-159.3 J/Kmol
= standard entropy change of the reaction =-159.3 J/Kmol