
Chemistry, 11.01.2020 03:31 eichlingkera3
3c2h2(g) → c6h6(g) what is the standard enthalphy change δho, for the reaction represented above? (δhof of c2h2(g) is 230 kj mol-1; (δhof of c6h6(g) is 83 kj mol-1; )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
3c2h2(g) → c6h6(g) what is the standard enthalphy change δho, for the reaction represented above? (...
Questions

Chemistry, 24.04.2020 01:56


Geography, 24.04.2020 01:56

Social Studies, 24.04.2020 01:56

Mathematics, 24.04.2020 01:56


Mathematics, 24.04.2020 01:56


History, 24.04.2020 01:56

English, 24.04.2020 01:57


Social Studies, 24.04.2020 01:57

History, 24.04.2020 01:57






French, 24.04.2020 01:57

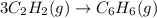
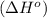 .
.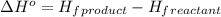
![\Delta H^o=[n_{C_6H_6}\times \Delta H_f^0_{(C_6H_6)}]-[n_{C_2H_2\times \Delta H_f^0_{(C_2H_2)}]](/tpl/images/0450/4494/c3760.png)
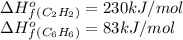
![\Delta H^o_{rxn}=[(1\times 83)]-[(3\times 230)]=-607kJ](/tpl/images/0450/4494/ed5f4.png)


