
Chemistry, 10.01.2020 06:31 aidanwindsor1738
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen to produce the oxide and then reduced with carbon to form elemental zinc and carbon monoxide. 2 zns + o2 2 zno + 2 so2 zno + c zn + co a crucible containing a sample of 0.50 mol zns was roasted in pure oxygen, then reduced with 1.00 mol carbon. what mass remained in the crucible after cooling?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen...
Questions


Chemistry, 14.07.2020 14:01

Chemistry, 14.07.2020 14:01




Mathematics, 14.07.2020 14:01


Mathematics, 14.07.2020 14:01


Mathematics, 14.07.2020 14:01





Biology, 14.07.2020 14:01

Geography, 14.07.2020 14:01



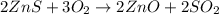 ..[1]
..[1]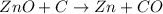 ..[2]
..[2]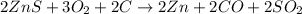 ..[3]
..[3]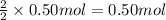 of Zn.
of Zn.


