Consider a solution containing 0.181 m lead ions and 0.365
miron(ii) ions. the ksp for lead su...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Questions


English, 13.01.2021 23:30


History, 13.01.2021 23:30

Health, 13.01.2021 23:30

English, 13.01.2021 23:30


Mathematics, 13.01.2021 23:30


Advanced Placement (AP), 13.01.2021 23:30

Mathematics, 13.01.2021 23:30

English, 13.01.2021 23:30

Mathematics, 13.01.2021 23:30

Health, 13.01.2021 23:30

Mathematics, 13.01.2021 23:30

Mathematics, 13.01.2021 23:30


English, 13.01.2021 23:30


![[H_3O^+]=6.8*10^{-22}](/tpl/images/0448/0948/8039f.png)
![[Pb^{+2}]=1*10^{-6}](/tpl/images/0448/0948/21ac4.png) :
:![Kps=[Pb^{+2}]*[S^{-2}]=3.4*10^{-28}](/tpl/images/0448/0948/0133d.png)
![1*10^{-6}*[S^{-2}]=3.4*10^{-28}](/tpl/images/0448/0948/5bca8.png)
![[S^{-2}]=3.4*10^{-22}](/tpl/images/0448/0948/c45b5.png)
![Kps=[0.365]*[S^{-2}]=3.7*10^{-19}](/tpl/images/0448/0948/c04d1.png)
![[S^{-2}]=1.01*10^{-18}](/tpl/images/0448/0948/5c3d9.png)
![[S^{-2}]](/tpl/images/0448/0948/d1351.png) concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate.
concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate. 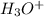
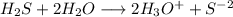
![[H_3O^+]=2*[S^{-2}]=6.8*10^{-22}](/tpl/images/0448/0948/3de14.png)


