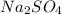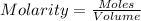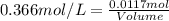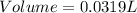Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
A5.05% (by mass) of aqueaus solution of sodium sulfate
isallowed to react with an excess of ba...
isallowed to react with an excess of ba...
Questions


History, 29.04.2021 14:00

Business, 29.04.2021 14:00


Mathematics, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00


Mathematics, 29.04.2021 14:00


Mathematics, 29.04.2021 14:00


Chemistry, 29.04.2021 14:00

Social Studies, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00

Social Studies, 29.04.2021 14:00

Mathematics, 29.04.2021 14:00


Computers and Technology, 29.04.2021 14:00

Chemistry, 29.04.2021 14:00

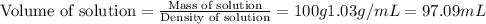
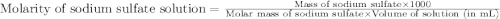
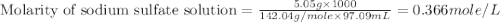
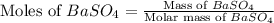
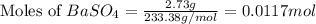
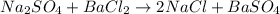
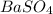 produced from 1 mole of
produced from 1 mole of