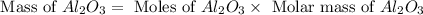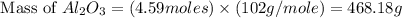Chemistry, 09.01.2020 06:31 josephmelichar777
The reaction betwen aluminum and iron (iii) oxide can
generatetemperatues approaching 3000 degrees celsius and is used in
weldingmetals:
2al + fe2o3
> al2o3 + 2 fe
in one process, 124 g of al are reacted with 601 g
offe2o3. calculate the mass in grams
ofal2o3 formed.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
The reaction betwen aluminum and iron (iii) oxide can
generatetemperatues approaching 3000 deg...
generatetemperatues approaching 3000 deg...
Questions

Biology, 19.09.2019 17:30





Social Studies, 19.09.2019 17:30


Mathematics, 19.09.2019 17:30

Biology, 19.09.2019 17:30

Health, 19.09.2019 17:30


Mathematics, 19.09.2019 17:30


Mathematics, 19.09.2019 17:30

Mathematics, 19.09.2019 17:30

Computers and Technology, 19.09.2019 17:30

Biology, 19.09.2019 17:30

World Languages, 19.09.2019 17:30


English, 19.09.2019 17:30

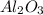 formed will be, 468.18 grams.
formed will be, 468.18 grams. 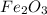 = 601 g
= 601 g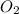 .
.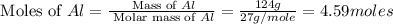
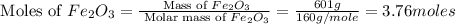
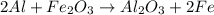
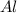 react with 1 mole of
react with 1 mole of 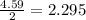 moles of
moles of 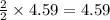 moles of
moles of