
Chemistry, 09.01.2020 06:31 Gribblejames
A0.1276 g sample of an unknown monoprotic acid was dissolved in 25.0 ml of water and titrated with 0.0633 m naoh solution. the volume of base required to bring the solution to the equivalence point was 18.4 ml. (a) calculate the molar mass of the acid. (b) after 10.0 ml of base had been added during the titration, the ph was determined to be 5.87. what is the ka of the unknown acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
A0.1276 g sample of an unknown monoprotic acid was dissolved in 25.0 ml of water and titrated with 0...
Questions

SAT, 27.04.2021 19:10

History, 27.04.2021 19:10

History, 27.04.2021 19:10

Social Studies, 27.04.2021 19:10



Mathematics, 27.04.2021 19:10


Mathematics, 27.04.2021 19:10

English, 27.04.2021 19:10

Chemistry, 27.04.2021 19:10







Mathematics, 27.04.2021 19:10


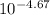 = 2.14 x 10⁻⁵
= 2.14 x 10⁻⁵

