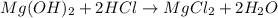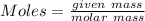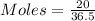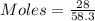Chemistry, 09.01.2020 06:31 deadpoolcorvettehats
In testing the effectiveness of an antacid compound, 20.0g of hydrochloric acid is mixed with 28.0g of magnesium hydroxide. will the base neutralize (completely use up) all of the acid? how much of which substance is in excess?
2hcl + (oh)2 -> mgcl2 + 2h20

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
In testing the effectiveness of an antacid compound, 20.0g of hydrochloric acid is mixed with 28.0g...
Questions

Social Studies, 04.12.2020 20:30

Geography, 04.12.2020 20:30


English, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30






History, 04.12.2020 20:30


Mathematics, 04.12.2020 20:30

History, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Social Studies, 04.12.2020 20:30


Social Studies, 04.12.2020 20:30