Solid potassium chlorate decomposes upon heating to form
solidpotassium chloride and oxygen ga...

Chemistry, 09.01.2020 06:31 lakinbacon4
Solid potassium chlorate decomposes upon heating to form
solidpotassium chloride and oxygen gas. a mixture of potassium
chlorateand potassium chloride with a mass of 7.44 g is heated
until allthe potassium chlorate has decomposed. if 789 ml of gas
iscolledcted over water at 0.976 atmospheres and 30.0
c, what is the percent potassium chlorate in theoriginal mixture.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Questions

Biology, 28.07.2019 02:30




Mathematics, 28.07.2019 02:30



Mathematics, 28.07.2019 02:30

English, 28.07.2019 02:30

History, 28.07.2019 02:30

Social Studies, 28.07.2019 02:30



Spanish, 28.07.2019 02:30


Biology, 28.07.2019 02:30




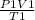 =
= 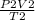
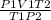
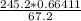 = 2.4232 g
= 2.4232 g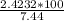 = 32.6%
= 32.6%

