The distribution constant for a molecule x between n-hexane and water
is 13.5. calculate the p...

Chemistry, 09.01.2020 06:31 jessixa897192
The distribution constant for a molecule x between n-hexane and water
is 13.5. calculate the percent of x remaining in the water phase that
was originally 0.0100 m in z after extraction of 50.0 ml of water with
4 extractions of 10.0 ml portions of n-hexane.

Answers: 3


Another question on Chemistry




Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Questions




Mathematics, 05.12.2019 23:31





History, 05.12.2019 23:31

Mathematics, 05.12.2019 23:31





History, 05.12.2019 23:31



Mathematics, 05.12.2019 23:31

Biology, 05.12.2019 23:31

![[X]_{i} = (\frac{V_{aq}}{V_{or}KD + V_{aq}})^{i} \cdot [X_{0}]](/tpl/images/0448/1496/650eb.png)
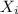 : is the concentration of A remaining in aqueous solution
: is the concentration of A remaining in aqueous solution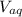 : is the volume of water
: is the volume of water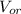 : is the volume of the organic solvent
: is the volume of the organic solvent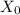 : is the original concentration of X
: is the original concentration of X![[X]_{i} = (\frac{50 mL}{10 mL \cdot 13.5 + 50 mL})^{4} \cdot 0.01 M = 5.34 \cdot 10^{-5} M](/tpl/images/0448/1496/4cebe.png)
![[X]_{i} = 5.34 \cdot 10^{-5} \cdot 100 \% = 0.005 \%](/tpl/images/0448/1496/48d0a.png)



