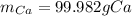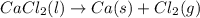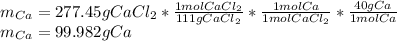Chemistry, 08.01.2020 12:31 sheldonwaid4278
Cacl2 can be melted to produce calcium metal and give off chlorine gas. the equation for this is cacl2(l) ca(s) + cl2(g). if 277.45 g cacl2 were melted, how many grams of ca(s) would be formed? (

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Cacl2 can be melted to produce calcium metal and give off chlorine gas. the equation for this is cac...
Questions


History, 05.05.2021 20:30

Mathematics, 05.05.2021 20:30

Mathematics, 05.05.2021 20:30

Chemistry, 05.05.2021 20:30

Mathematics, 05.05.2021 20:30


Mathematics, 05.05.2021 20:30


Mathematics, 05.05.2021 20:30



Mathematics, 05.05.2021 20:30

Mathematics, 05.05.2021 20:30


History, 05.05.2021 20:30

World Languages, 05.05.2021 20:30



Mathematics, 05.05.2021 20:30