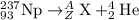Identify the nuclide produced when neptunium-237 decays by alpha emission:
237 93np→42h...

Chemistry, 08.01.2020 00:31 jazzzzhands21
Identify the nuclide produced when neptunium-237 decays by alpha emission:
237 93np→42he + ?
express your answer as an isotope using prescripts.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
Questions

Social Studies, 13.10.2020 15:01






Biology, 13.10.2020 15:01

History, 13.10.2020 15:01

Health, 13.10.2020 15:01


Chemistry, 13.10.2020 15:01

Computers and Technology, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Business, 13.10.2020 15:01



Chemistry, 13.10.2020 15:01



History, 13.10.2020 15:01

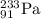
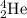 is being emitted.
is being emitted.