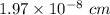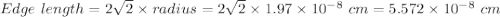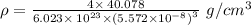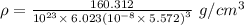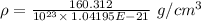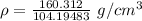Chemistry, 06.01.2020 18:31 karlamiddleschool
Calcium has a cubic closest packed structure as a solid. assuming that calcium has an atomic radius of 197 pm, calculate the density of solid calcium.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Calcium has a cubic closest packed structure as a solid. assuming that calcium has an atomic radius...
Questions

Mathematics, 08.10.2019 18:30

Mathematics, 08.10.2019 18:30

Health, 08.10.2019 18:30

Advanced Placement (AP), 08.10.2019 18:30


Biology, 08.10.2019 18:30

Computers and Technology, 08.10.2019 18:30

History, 08.10.2019 18:30




Mathematics, 08.10.2019 18:30

History, 08.10.2019 18:30

Health, 08.10.2019 18:30

Mathematics, 08.10.2019 18:30

Business, 08.10.2019 18:30

History, 08.10.2019 18:30



History, 08.10.2019 18:30

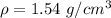
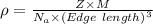

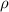 is the density
is the density