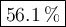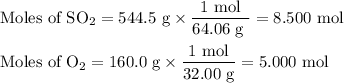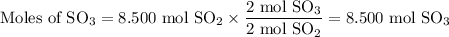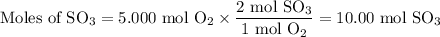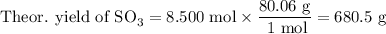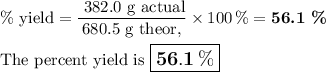Chemistry, 05.01.2020 19:31 johnandashley5p65r4a
What is the percent yield of the reaction below when 544.5 g so2 and 160.0g o2 produce 382.0 g so3? 2so2+o2+2so3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
What is the percent yield of the reaction below when 544.5 g so2 and 160.0g o2 produce 382.0 g so3?...
Questions


Mathematics, 05.11.2020 15:40

Arts, 05.11.2020 15:40

Mathematics, 05.11.2020 15:40

Mathematics, 05.11.2020 15:40

English, 05.11.2020 15:40

Mathematics, 05.11.2020 15:40

Mathematics, 05.11.2020 15:40

English, 05.11.2020 15:40

English, 05.11.2020 15:40

Physics, 05.11.2020 15:40

Mathematics, 05.11.2020 15:40


Biology, 05.11.2020 15:40



Computers and Technology, 05.11.2020 15:40

Biology, 05.11.2020 15:40


Mathematics, 05.11.2020 15:40