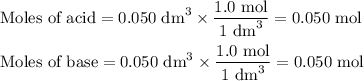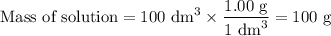Chemistry, 05.01.2020 17:31 gharrell03
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorimeter. the temperature of the calorimeter rose by 4.5°c. calculate the heat of reaction per mol of h20 formed.( heat capacity of the calorimeter is 50j/°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorime...
Questions

History, 02.10.2019 17:00

Chemistry, 02.10.2019 17:00


Mathematics, 02.10.2019 17:00

Biology, 02.10.2019 17:00

Mathematics, 02.10.2019 17:00

Biology, 02.10.2019 17:00


Social Studies, 02.10.2019 17:00


History, 02.10.2019 17:00


Biology, 02.10.2019 17:00



Mathematics, 02.10.2019 17:00


Mathematics, 02.10.2019 17:00


Mathematics, 02.10.2019 17:00