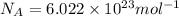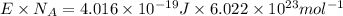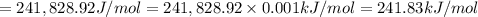Chemistry, 03.01.2020 20:31 abraralzaher
The longest wavelength of light with enough energy to break the cl-cl bond in cl2(g) is 495 nm.
1) calculate the frequency in s-1 of the light2) calculate the energy in j of a photon of the light3) calculate the minimun energy in kj mol-1 of the cl-cl bond

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
The longest wavelength of light with enough energy to break the cl-cl bond in cl2(g) is 495 nm.
Questions




Geography, 30.08.2021 17:40




Mathematics, 30.08.2021 17:40


Mathematics, 30.08.2021 17:40

Chemistry, 30.08.2021 17:40

Mathematics, 30.08.2021 17:40

Mathematics, 30.08.2021 17:40

Mathematics, 30.08.2021 17:40

Chemistry, 30.08.2021 17:40

Mathematics, 30.08.2021 17:40

History, 30.08.2021 17:40


Chemistry, 30.08.2021 17:40

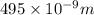
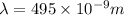
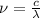
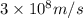
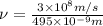
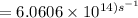
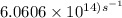 .
.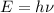
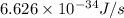
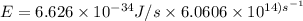

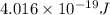 .
.