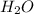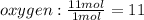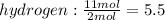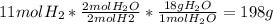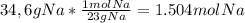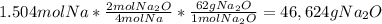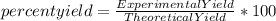a) write the complete balanced equation.

1) hydrogen and oxygen react to form water.
a) write the complete balanced equation.
b) if 11 moles of hydrogen react with 11 moles of oxygen which of these is the limiting reagent? show work or explain how you know.
c) what is the maximum amount of water (in grams) that can be produced given 11 moles of hydrogen and 11 moles of oxygen? show work.
2) sodium reacts with oxygen to produce sodium oxide as described by the balanced equation below. if 34.6g of sodium reacts with excess oxygen gas to produce 41.8g of sodium oxide, what is the percent yield? show all work. (hint: be sure to calculate theoretical yield first)
4na + o2 --> 2na2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

You know the right answer?
1) hydrogen and oxygen react to form water.
a) write the complete balanced equation.
a) write the complete balanced equation.
Questions












Biology, 19.09.2019 23:00








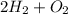 ⇒
⇒ 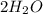
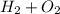 ⇒
⇒