Chemistry, 03.01.2020 03:31 lucywood2024
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the product gas, which contains 50.0 mole% h2, 40.0% , and the balance emerges at 500°c at a rate of 2.50m3/h and goes to a condenser. the gas and liquid streams leaving the condenser are in equilibrium at 15°c and 1 bar. the liquid may be taken to be pure w"a) calculate the % excess steam fed to the reactor and rate of condensation of the water (kg/h) leaving the condensor. b) calculate the rate (kw) at which heat must be removed from the condensor. c) calculate the rate of heat transfer (kw) to or from the reactor (state which it is).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

Chemistry, 23.06.2019 13:40
Match these items with their examples. 1. liquid solution milk 2. solid solution aluminum foil 3. compound soda 4. colloid steel 5. element salt
Answers: 1

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the prod...
Questions



Mathematics, 09.03.2021 01:20



Computers and Technology, 09.03.2021 01:20

English, 09.03.2021 01:20

Health, 09.03.2021 01:20

Mathematics, 09.03.2021 01:20


Mathematics, 09.03.2021 01:20



Mathematics, 09.03.2021 01:20

Mathematics, 09.03.2021 01:20

Mathematics, 09.03.2021 01:20


Mathematics, 09.03.2021 01:20


Computers and Technology, 09.03.2021 01:20

 kW
kW
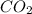 = 40 moles
= 40 moles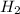 = 40 moles
= 40 moles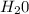 = 20 moles
= 20 moles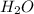
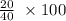 %
%


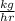

 (-16324.231)
(-16324.231)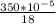 (-16324.231) *
(-16324.231) *