Chemistry, 03.01.2020 02:31 oliviaparker2010
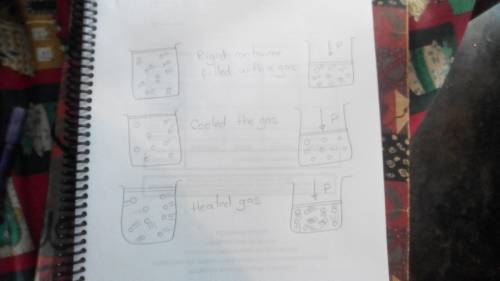
Draw a highly magnified view of a sealed, rigid container filled with a gas. then draw what it would look like if you cooled the gas significantly but kept the temperature above the boiling point of the substance in the container. also draw what it would look like if you heated the gas significantly. finally, draw what each situation would look like if you evacuated enough of the gas to decrease the pressure by a factor of 2.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Draw a highly magnified view of a sealed, rigid container filled with a gas. then draw what it would...
Questions


Mathematics, 19.07.2019 10:00


English, 19.07.2019 10:00


Mathematics, 19.07.2019 10:00

History, 19.07.2019 10:00

Mathematics, 19.07.2019 10:00

Computers and Technology, 19.07.2019 10:00


Chemistry, 19.07.2019 10:00

Geography, 19.07.2019 10:00


Mathematics, 19.07.2019 10:00

Mathematics, 19.07.2019 10:00

History, 19.07.2019 10:00