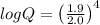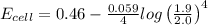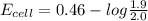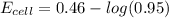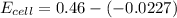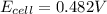Chemistry, 02.01.2020 23:31 JellalFernandes
Avoltaic cell utilizes the following reaction: 4fe2+(aq)+o2(g)+4h+(aq)→4fe3+(aq) +2h2o(l).
the emf under standard contions is .46 v.
what is the emf of this cell when [fe2+]= 2.0m , [fe3+]= 1.9

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
Avoltaic cell utilizes the following reaction: 4fe2+(aq)+o2(g)+4h+(aq)→4fe3+(aq) +2h2o(l).
...
...
Questions


Physics, 19.08.2019 10:00

History, 19.08.2019 10:00

History, 19.08.2019 10:00


Biology, 19.08.2019 10:00

Social Studies, 19.08.2019 10:00

Biology, 19.08.2019 10:00

Mathematics, 19.08.2019 10:00

Social Studies, 19.08.2019 10:00



Biology, 19.08.2019 10:00

Mathematics, 19.08.2019 10:00


History, 19.08.2019 10:00

Mathematics, 19.08.2019 10:00


History, 19.08.2019 10:00

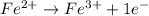 ......E = 0.77 V
......E = 0.77 V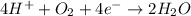 ....E= 1.23 V
....E= 1.23 V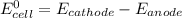
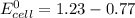
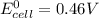
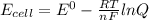
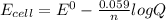
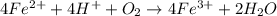
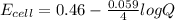
![log Q = \frac{[Fe^{3+}]^{4}}{[Fe^{2+}]^{4}}](/tpl/images/0440/3897/ea23c.png)
![log Q = \left ( \frac{[Fe^{3+}]}{[Fe^{2+}]} \right )^{4}](/tpl/images/0440/3897/409de.png)