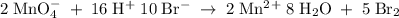Chemistry, 02.01.2020 19:31 canyonrico05
2mno4-(aq) + 10 br-(aq) + 16 h+(aq) → 2 mn2+(aq) + 5 br2(aq) + 8 h2o(l). how many electrons are transferred in the reaction represented by the balanced equation above?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
Aluminum–lithium (al-li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. a commercial aircraft skin material having a density of 2.47 g/cm3 is desired. compute the concentration of li (in wt%) that is required.
Answers: 3

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
2mno4-(aq) + 10 br-(aq) + 16 h+(aq) → 2 mn2+(aq) + 5 br2(aq) + 8 h2o(l). how many electrons are tran...
Questions



Mathematics, 06.05.2020 03:22

Mathematics, 06.05.2020 03:22

Mathematics, 06.05.2020 03:22

Chemistry, 06.05.2020 03:22

Biology, 06.05.2020 03:22

Physics, 06.05.2020 03:22





Geography, 06.05.2020 03:22

English, 06.05.2020 03:22


Biology, 06.05.2020 03:22




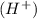 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.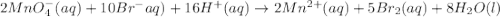
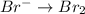
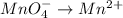
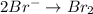
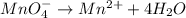
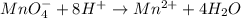
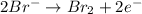
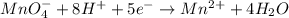
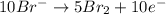
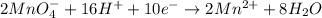
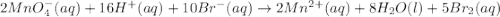
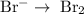
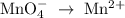
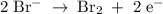
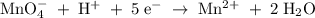 .
.