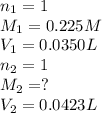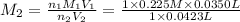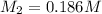Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
Suppose you have a 0.0423 l sample of koh of unknown concentration. if this amount of koh is used to...
Questions


Mathematics, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00


Health, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

Social Studies, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00


English, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

English, 05.02.2021 03:00


History, 05.02.2021 03:00

English, 05.02.2021 03:00




Mathematics, 05.02.2021 03:00

Mathematics, 05.02.2021 03:00

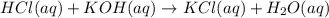
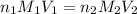
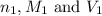 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 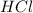
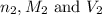 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.