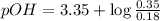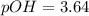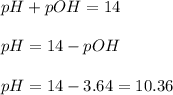Chemistry, 31.12.2019 05:31 acostasilvana1osw8tk
Asolution is prepared at that is initially in methylamine , a weak base with , and in methylammonium chloride . calculate the ph of the solution. round your answer to decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Asolution is prepared at that is initially in methylamine , a weak base with , and in methylammonium...
Questions


Chemistry, 02.02.2020 19:47

History, 02.02.2020 19:47

Mathematics, 02.02.2020 19:47

English, 02.02.2020 19:47

Mathematics, 02.02.2020 19:47

Biology, 02.02.2020 19:47










Biology, 02.02.2020 19:47

English, 02.02.2020 19:47

Chemistry, 02.02.2020 19:47

Mathematics, 02.02.2020 19:47

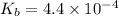 , and 0.35 M in methylammonium bromide (CH₃NH₃Br). Calculate the pH of the solution. Round your answer to 2 decimal places.
, and 0.35 M in methylammonium bromide (CH₃NH₃Br). Calculate the pH of the solution. Round your answer to 2 decimal places.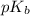 .
.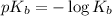
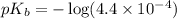
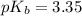
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0438/0622/ac570.png)
![pOH=pK_b+\log \frac{[CH_3NH_3Br]}{[CH_3NH_2]}](/tpl/images/0438/0622/9ec0e.png)