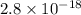Chemistry, 31.12.2019 04:31 babygirl10302015
The solubility product ag3po4 is: ksp = 2.8 x 10^-18. what is the solubility of ag3po4 in water, in moles per liter?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2


You know the right answer?
The solubility product ag3po4 is: ksp = 2.8 x 10^-18. what is the solubility of ag3po4 in water, in...
Questions

Mathematics, 20.09.2020 02:01




English, 20.09.2020 02:01



French, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01






Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

History, 20.09.2020 02:01

History, 20.09.2020 02:01

English, 20.09.2020 02:01

Biology, 20.09.2020 02:01

 in water is,
in water is, 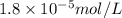

![K_{sp}=[Ag^{+}]^3[PO_4^{3-}]](/tpl/images/0437/9779/7aeef.png)
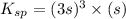

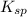 =
=